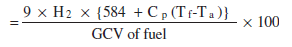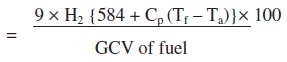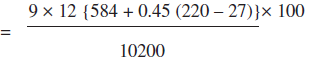BOILERS
Syllabus
Boilers: Types, Combustion in boilers, Performances evaluation, Analysis of losses, Feed water treatment, Blow down, Energy conservation opportunities.
2.1 Introduction
A boiler is an enclosed vessel that provides a means for combustion heat to be transferred into water until it becomes heated water or steam. The hot water or steam under pressure is then usable for transferring the heat to a process. Water is a useful and cheap medium for transferring heat to a process. When water is boiled into steam its volume increases about 1,600 times, producing a force that is almost as explosive as gunpowder. This causes the boiler to be extremely dangerous equipment that must be treated with utmost care.
The process of heating a liquid until it reaches its gaseous state is called evaporation. Heat is transferred from one body to another by means of (1) radiation, which is the transfer of heat from a hot body to a cold body without a conveying medium, (2) convection, the transfer of heat by a conveying medium, such as air or water and (3) conduction, transfer of heat by actual physical contact, molecule to molecule.
Boiler Specification
Typical Boiler Specification
| Boiler Make & Year | : XYZ & 2003 |
| MCR(Maximum Continuous Rating) | : 10TPH (F & A 100oC) |
| Rated Working Pressure | : 10.54 kg/cm2(g) |
| Type of Boiler | : 3 Pass Fire tube |
| Fuel Fired | : Fuel Oil |
The heating surface is any part of the boiler metal that has hot gases of combustion on one side and water on the other. Any part of the boiler metal that actually contributes to making steam is heating surface. The amount of heating surface of a boiler is expressed in square meters. The larger the heating surface a boiler has, the more efficient it becomes. The quantity of the steam produced is indicated in tons of water evaporated to steam per hour. Maximum continuous rating is the hourly evaporation that can be maintained for 24 hours. F & A means the amount of steam generated from water at 100oC to saturated steam at 100oC.
Indian Boiler Regulation
The Indian Boilers Act was enacted to consolidate and amend the law relating to steam boilers. Indian Boilers Regulation (IBR) was created in exercise of the powers conferred by section 28 & 29 of the Indian Boilers Act.
IBR Steam Boilers means any closed vessel exceeding 22.75 liters in capacity and which is used expressively for generating steam under pressure and includes any mounting or other fitting attached to such vessel, which is wholly, or partly under pressure when the steam is shut off.
IBR Steam Pipe means any pipe through which steam passes from a boiler to a prime mover or other user or both, if pressure at which steam passes through such pipes exceeds 3.5 kg/cm2 above atmospheric pressure or such pipe exceeds 254 mm in internal diameter and includes in either case any connected fitting of a steam pipe.
2.2 Boiler Systems
The boiler system comprises of: feed water system, steam system and fuel system. The feed water system provides water to the boiler and regulates it automatically to meet the steam demand. Various valves provide access for maintenance and repair. The steam system collects and controls the steam produced in the boiler. Steam is directed through a piping system to the point of use. Throughout the system, steam pressure is regulated using valves and checked with steam pressure gauges. The fuel system includes all equipment used to provide fuel to generate the necessary heat. The equipment required in the fuel system depends on the type of fuel used in the system. A typical boiler room schematic is shown in Figure 2.1.
Figure 2.1 Boiler Room Schematic
The water supplied to the boiler that is converted into steam is called feed water. The two sources of feed water are: (1) feed water or condensed steam returned from the processes and (2) Makeup water (treated raw water) which must come from outside the boiler room and plant processes. For higher boiler efficiencies, the feed water is preheated by economizer, using the waste heat in the flue gas.
2.3 Boiler Types and Classifications
There are virtually infinite numbers of boiler designs but generally they fit into one of two categories:
The features of package boilers are:
- Small combustion space and high heat release rate resulting in faster evaporation.
- Large number of small diameter tubes leading to good convective heat transfer.
- Forced or induced draft systems resulting in good combustion efficiency.
- Number of passes resulting in better overall heat transfer.
- Higher thermal efficiency levels compared with other boilers.
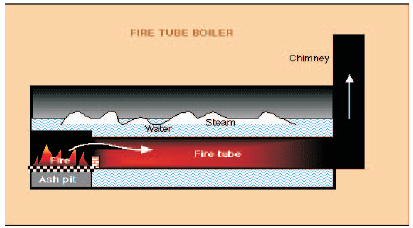
These boilers are classified based on the number of passes - the number of times the hot combustion gases pass through the boiler. The combustion chamber is taken, as the first pass after which there may be one, two or three sets of fire-tubes. The most common boiler of this class is a three-pass unit with two sets of fire-tubes and with the exhaust gases exiting through the rear of the boiler.
Stoker Fired Boiler:
Stokers are classified according to the method of feeding fuel to the furnace and by the type of grate. The main classifications are:
- Chain-grate or traveling-grate stoker
- Spreader stoker
Chain-Grate or Traveling-Grate Stoker Boiler
Coal is fed onto one end of a moving steel chain grate. As grate moves along the length of the furnace, the coal burns before dropping off at the end as ash. Some degree of skill is required, particularly when setting up the grate, air dampers and baffles, to ensure clean combustion leaving minimum of unburnt carbon in the ash.
Figure 2.5 Chain Grate Stoker
The coal-feed hopper runs along the entire coal-feed end of the furnace. Acoal grate is used to control the rate at which coal is fed into the furnace, and to control the thickness of the coal bed and speed of the grate. Coal must be uniform in size, as large lumps will not burn out completely by the time they reach the end of the grate. As the bed thickness decreases from coalfeed end to rear end, different amounts of air are required- more quantity at coal-feed end and less at rear end (see Figure 2.5).
Spreader Stoker Boiler
Spreader stokers (see figure 2.6) utilize a combination of suspension burning and grate burning. The coal is continually fed into the furnace above a burning bed of coal. The coal fines are burned in suspension; the larger particles fall to the grate, where they are burned in a thin, fastburning coal bed. This method of firing provides good flexibility to meet load fluctuations, since ignition is almost instantaneous when firing rate is increased. Hence, the spreader stoker is favored over other types of stokers in many industrial applications.
Figure 2.6 Spreader Stoker
Pulverized Fuel Boiler
Most coal-fired power station boilers use pulverized coal, and many of the larger industrial water-tube boilers also use this pulverized fuel. This technology is well developed, and there are thousands of units around the world, accounting for well over 90% of coal-fired capacity.
The coal is ground (pulverised) to a fine powder, so that less than 2% is +300 micro metre (μm) and 70-75% is below 75 microns, for a bituminous coal. It should be noted that too fine a powder is wasteful of grinding mill power. On the other hand, too coarse a powder does not burn completely in the combustion chamber and results in higher unburnt losses.
FBC Boiler
Fluidised bed combustion has significant advantages over conventional firing systems and offers multiple benefits namely fuel flexibility, reduced emission of noxious pollutants such as SOx and NOx, compact boiler design and higher combustion efficiency. More details about FBC boilers are given in Chapter 6 on Fluidized Bed Boiler.
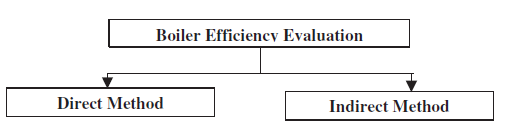
2.4 Performance Evaluation of Boilers
The performance parameters of boiler, like efficiency and evaporation ratio reduces with time due to poor combustion, heat transfer surface fouling and poor operation and maintenance. Even for a new boiler, reasons such as deteriorating fuel quality, water quality etc. can result in poor boiler performance. Boiler efficiency tests help us to find out the deviation of boiler efficiency from the best efficiency and target problem area for corrective action.
Boiler Efficiency
Thermal efficiency of boiler is defined as the percentage of heat input that is effectively utilised to generate steam. There are two methods of assessing boiler efficiency.
- The Direct Method: Where the energy gain of the working fluid (water and steam) is compared with the energy content of the boiler fuel.
- The Indirect Method: Where the efficiency is the difference between the losses and the energy input.
a. Direct Method
This is also known as ‘input-output method’ due to the fact that it needs only the useful output (steam) and the heat input (i.e. fuel) for evaluating the efficiency. This efficiency can be evaluated using the formula
Parameters to be monitored for the calculation of boiler efficiency by direct method are :
- Quantity of steam generated per hour (Q) in kg/hr.
- Quantity of fuel used per hour (q) in kg/hr.
- The working pressure (in kg/cm2(g)) and superheat temperature (oC), if any
- The temperature of feed water (oC)
- Type of fuel and gross calorific value of the fuel (GCV) in kCal/kg of fuel
| where, | hg - Enthalpy of saturated steam in kCal/kg of steam |
| hf - Enthalpy of feed water in kCal/kg of water |
Example
Find out the efficiency of the boiler by direct method with the data given below:
| - Type of boiler | : Coal fired |
| - Quantity of steam (dry) generated | : 8 TPH |
| - Steam pressure (gauge) / temp | : 10 kg/cm2(g)/ 180oC |
| - Quantity of coal consumed | : 1.8 TPH |
| - Feed water temperature | : 85oC |
| - GCV of coal | : 3200 kCal/kg |
| - Enthalpy of steam at 10 kg/cm2 pressure | : 665 kCal/kg (saturated) |
| - Enthalpy of feed water | : 85 kCal/kg |
It should be noted that boiler may not generate 100% saturated dry steam, and there may be some amount of wetness in the steam.
Advantages of direct method:
- Plant people can evaluate quickly the efficiency of boilers
- Requires few parameters for computation
- Needs few instruments for monitoring
Disadvantages of direct method:
- Does not give clues to the operator as to why efficiency of system is lower
- Does not calculate various losses accountable for various efficiency levels
b. Indirect Method
There are reference standards for Boiler Testing at Site using indirect method namely British Standard, BS 845: 1987 and USA Standard is ASME PTC-4-1 Power Test Code Steam Generating Units'.
Indirect method is also called as heat loss method. The efficiency can be arrived at, by subtracting the heat loss fractions from 100. The standards do not include blow down loss in the efficiency determination process. A detailed procedure for calculating boiler efficiency by indirect method is given below. However, it may be noted that the practicing energy mangers in industries prefer simpler calculation procedures.
The principle losses that occur in a boiler are:
- Loss of heat due to dry fluegas
- Loss of heat due to moisture in fuel and combustion air
- Loss of heat due to combustion of hydrogen
- Loss of heat due to radiation
- Loss of heat due to unburnt
In the above, loss due to moisture in fuel and the loss due to combustion of hydrogen are dependent on the fuel, and cannot be controlled by design.
The data required for calculation of boiler efficiency using indirect method are:
- Ultimate analysis of fuel (H2, O2, S, C, moisture content, ash content)
- Percentage of Oxygen or CO2 in the flue gas
- Flue gas temperature in oC (Tf )
- Ambient temperature in oC (Ta) & humidity of air in kg/kg of dry air
- GCV of fuel in kCal/kg
- Percentage combustible in ash (in case of solid fuels)
- GCV of ash in kCal/kg (in case of solid fuels)
Solution :
Theoretical air requirement
=[(11.6 × C) + {34.8 × (H2 - O2/8)} + (4.35 × S)]/100 kg/kg of fuel
| Excess Air supplied (EA) = |
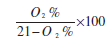
|
Actual mass of air supplied/ kg of fuel (AAS) = {1 + EA/100} × theoretical air
| i. Percentage heat loss due to dry flue gas = |
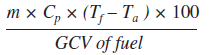
|
- m = mass of dry flue gas in kg/kg of fuel
- m = Combustion products from fuel: CO2 + SO2 + Nitrogen in fuel + Nitrogen in the actual mass of air supplied + O2 in flue gas. (H2O/Water vapour in the flue gas should not be considered)
- Cp = Specific heat of flue gas (0.23 kCal/kg oC)
ii. Percentage heat loss due to evaporation of water formed due to H2 in fuel
Where, H2 - kg of H2 in 1 kg of fuel
Cp - Specific heat of superheated steam (0.45 kCal/kg oC)
iii. Percentage heat loss due to evaporation of moisture present in fuel
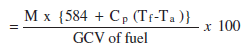
| Where, | M - kg of moisture in 1kg of fuel |
| Cp - Specific heat of superheated steam (0.45 kCal/kg)oC | |
| 584 is the latent heat corresponding to the partial pressure of water vapour. |
iv. Percentage heat loss due to moisture present in air

Cp - Specific heat of superheated steam (0.45 kCal/kg oC)
v. Percentage heat loss due to unburnt in fly ash
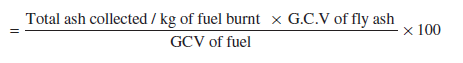
vi. Percentage heat loss due to unburnt in bottom ash

vii. Percentage heat loss due to radiation and other unaccounted loss
The actual radiation and convection losses are difficult to assess because of particular emissivity of various surfaces, its inclination, air flow pattern etc. In a relatively small boiler, with a capacity of 10 MW, the radiation and unaccounted losses could amount to between 1% and 2% of the gross calorific value of the fuel, while in a 500 MW boiler, values between 0.2% to 1% are typical. The loss may be assumed appropriately depending on the surface condition.
Example: The following are the data collected for a typical oil fired boiler. Find out the efficiency of the boiler by indirect method and Boiler Evaporation ratio.
| • | Type of boiler | : Oil fired |
| • | Ultimate analysis of Oil C : 84.0 % S : 3.0 % | H2 : 12.0 % O2 : 1.0 % |
| • | GCV of Oil | : 10200 kCal/kg |
| • | Steam Generation Pressure | : 7kg/cm2(g)-saturated |
| • | Enthalpy of steam | : 660 kCal/kg |
| • | Feed water temperature | : 60 oC |
| • | Percentage of Oxygen in flue gas | : 7 |
| • | Percentage of CO2 in flue gas | : 11 |
| • | Flue gas temperature (Tf) | : 220 oC |
| • | Ambient temperature (Ta) | : 27 oC |
| • | Humidity of air | : 0.018 kg/kg of dry air |
Solution
Step-1: Find the theoretical air requirement
= [(11.6 × C) +{34.8 × (H2 - O2 /8)} +(4.35 × S)] /100 kg/kg of oil
=[(11.6 × 84) + [{34.8 × (12 - 1/8)} + (4.35 × 3)]/100 kg/kg of oil
=14 kg of air/kg of oil
Step-2: Find the %Excess air supplied
| Excess air supplied (EA) | = (O2 × 100)/(21-O2) |
| = (7 × 100)/(21-7) | |
| = 50% |
Step-3: Find the Actual mass of air supplied
| Actual mass of air supplied /kg of fuel (AAS) | = [ 1 + EA/100] × Theoritical Air |
| = [1 + 50/100] × 14 | |
| = 1.5 × 14 | |
| = 21 kg of air/kg of oil |
Step-4: Estimation of all losses
i. Dry flue gas loss
| Percentage heat loss due to dry flue gas = |
 /a> /a>
|
m= mass of CO2 + mass of SO2 + mass of N2 + mass of O2

m = 21 kg / kg of oil
| Percentage heat loss due to dry flue gas |

|
Alternatively a simple method can be used for determining the dry flue gas loss as given below.
| a) Percentage heat loss due to dry flue gas |

|
| Total mass of flue gas (m) | = mass of actual air supplied + mass of fuel supplied |
| = 21 + 1 = 22 | |
| %Dry flue gas loss |
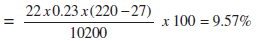
|
ii. Heat loss due to evaporation of water formed due to H2 in fuel
iii. Heat loss due to moisture present in air
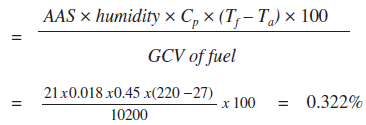
iv. Heat loss due to radiation and other unaccounted losses
For a small boiler it is estimated to be 2%
Boiler Efficiency
| i. | Heat loss due to dry flue gas | : 9.14% |
| ii. | Heat loss due to evaporation of water formed due to H2 in fuel | : 7.10 % |
| iii. | Heat loss due to moisture present in air | : 0.322 % |
| iv. | Heat loss due to radiation and other unaccounted loss | : 2% |
| Boiler Efficiency | = 100 - [9.14 + 7.10 + 0.322 + 2] |
| = 100 - 18.56 = 81 %(app) |
| Evaporation Ratio | = Heat utilised for steam generation/Heat addition to the steam |
| = 10200 × 0.83/ (660-60) | |
| = 14.11 |
Boiler Evaporation Ratio
Evaporation ratio means kilogram of steam generated per kilogram of fuel consumed.
| Typical Examples: | Coal fired boiler: 6 |
| Oil fired boiler: 13 | |
| i.e 1 kg of coal can generate 6 kg of steam | |
| 1 kg of oil can generate 13 kg of steam |
However, this figure will depend upon type of boiler, calorific value of the fuel and associated efficiencies.
2.5 Boiler Blowdown
When water is boiled and steam is generated, any dissolved solids contained in the water remain in the boiler. If more solids are put in with the feed water, they will concentrate and may eventually reach a level where their solubility in the water is exceeded and they deposit from the solution. Above a certain level of concentration, these solids encourage foaming and cause carryover of water into the steam. The deposits also lead to scale formation inside the boiler, resulting in localized overheating and finally causing boiler tube failure.
It is, therefore, necessary to control the level of concentration of the solids and this is achieved by the process of 'blowing down', where a certain volume of water is blown off and is automatically replaced by feed water - thus maintaining the optimum level of total dissolved solids (TDS) in the boiler water. Blow down is necessary to protect the surfaces of the heat exchanger in the boiler. However, blow down can be a significant source of heat loss, if improperly carried out. The maximum amount of total dissolved solids (TDS) concentration permissible in various types of boilers is given in Table 2.1.
TABLE 2.1 RECOMMENDED TDS LEVELS FOR VARIOUS BOILERS | ||
| Boiler Type | Maximum TDS (ppm)* | |
| 1. | Lancashire | 10,000 ppm |
| 2. | Smoke and water tube boilers (12 kg/cm2) | 5,000 ppm |
| 3. | Low pressure Water tube boiler | 2000-3000 |
| 4. | High Pressure Water tube boiler with superheater etc. | 3,000-3,500 ppm |
| 5. | Package and economic boilers | 3,000 ppm |
| 6. | Coil boilers and steam generators | 2000 (in the feed water |
Note: Refer guidelines specified by manufacturer for more details
*parts per million
Conductivity as Indicator of Boiler Water Quality
Since it is tedious and time consuming to measure total dissolved solids (TDS) in boiler water system, conductivity measurement is used for monitoring the overall TDS present in the boiler. A rise in conductivity indicates a rise in the "contamination" of the boiler water.
Conventional methods for blowing down the boiler depend on two kinds of blowdown – intermittent and continuous
Intermittent Blowdown
The intermittent blown down is given by manually operating a valve fitted to discharge pipe at the lowest point of boiler shell to reduce parameters (TDS or conductivity, pH, Silica and Phosphates concentration) within prescribed limits so that steam quality is not likely to be affected. In intermittent blowdown, a large diameter line is opened for a short period of time, the time being based on a thumb rule such as "once in a shift for 2 minutes".
Intermittent blowdown requires large short-term increases in the amount of feed water put into the boiler, and hence may necessitate larger feed water pumps than if continuous blow down is used. Also, TDS level will be varying, thereby causing fluctuations of the water level in the boiler due to changes in steam bubble size and distribution which accompany changes in concentration of solids. Also substantial amount of heat energy is lost with intermittent blowdown.
Continuous Blowdown
There is a steady and constant dispatch of small stream of concentrated boiler water, and replacement by steady and constant inflow of feed water. This ensures constant TDS and steam
Figure 2.9 Blowdown Heat Recovery System
purity at given steam load. Once blow down valve is set for a given conditions, there is no need for regular operator intervention
Even though large quantities of heat are wasted, opportunity exists for recovering this heat by blowing into a flash tank and generating flash steam. This flash steam can be used for preheating boiler feed water or for any other purpose (see Figure 2.9 for blow down heat recovery system). This type of blow down is common in high-pressure boilers.
Blowdown calculations
The quantity of blowdown required to control boiler water solids concentration is calculated by using the following formula:
| Blow down (%) = | Feed water TDS × % Make up water |
| Maximum Permissible TDS in Boiler water |
If maximum permissible limit of TDS as in a package boiler is 3000 ppm, percentage make up water is 10% and TDS in feed water is 300 ppm, then the percentage blow down is given as:
= 300 x 10/ 3000
= 1%
If boiler evaporation rate is 3000 kg/hr then required blow down rate is:
| 3000 × 1 |
| 100 |
= 30 kg/hr
Benefits of Blowdown
Good boiler blow down control can significantly reduce treatment and operational costs that include:
- - Lower pretreatment costs
- - Less make-up water consumption
- - Reduced maintenance downtime
- - Increased boiler life
- - Lower consumption of treatment chemicals
2.6 Boiler Water Treatment
Producing quality steam on demand depends on properly managed water treatment to control steam purity, deposits and corrosion. A boiler is the sump of the boiler system. It ultimately receives all of the pre-boiler contaminants. Boiler performance, efficiency, and service life are direct products of selecting and controlling feed water used in the boiler.
When feed water enters the boiler, the elevated temperatures and pressures cause the components of water to behave differently. Most of the components in the feed water are soluble. However, under heat and pressure most of the soluble components come out of solution as particulate solids, sometimes in crystallized forms and other times as amorphous particles. When solubility of a specific component in water is exceeded, scale or deposits develop. The boiler water must be sufficiently free of deposit forming solids to allow rapid and efficient heat transfer and it must not be corrosive to the boiler metal.
Deposit Control
Deposits in boilers may result from hardness contamination of feed water and corrosion products from the condensate and feed water system. Hardness contamination of the feed water may arise due to deficient softener system.
Deposits and corrosion result in efficiency losses and may result in boiler tube failures and inability to produce steam. Deposits act as insulators and slows heat transfer. Large amounts of deposits throughout the boiler could reduce the heat transfer enough to reduce the boiler efficiency significantly. Different type of deposits affects the boiler efficiency differently. Thus it may be useful to analyse the deposits for its characteristics. The insulating effect of deposits causes the boiler metal temperature to rise and may lead to tube-failure by overheating.
Impurities Causing Deposits
The most important chemicals contained in water that influences the formation of deposits in the boilers are the salts of calcium and magnesium, which are known as hardness salts.
Calcium and magnesium bicarbonate dissolve in water to form an alkaline solution and these salts are known as alkaline hardness. They decompose upon heating, releasing carbon dioxide and forming a soft sludge, which settles out. These are called temporary hardness-hardness that can be removed by boiling
Calcium and magnesium sulphates, chlorides and nitrates, etc. when dissolved in water are chemically neutral and are known as non-alkaline hardness. These are called permanent hardness and form hard scales on boiler surfaces, which are difficult to remove. Non-alkalinity hardness chemicals fall out the solution due to reduction in solubility as the temperature rises, by concentration due to evaporation which takes place within the boiler, or by chemical change to a less soluble compound.
Silica
The presence of silica in boiler water can rise to formation of hard silicate scales. It can also associate with calcium and magnesium salts, forming calcium and magnesium silicates of very low thermal conductivity. Silica can give rise to deposits on steam turbine blades, after been carried over either in droplets of water in steam, or in volatile form in steam at higher pressures.
Two major types of boiler water treatment are: Internal water treatment and External water treatment.
Internal Water Treatment
Internal treatment is carried out by adding chemicals to boiler to prevent the formation of scale by converting the scale-forming compounds to free-flowing sludges, which can be removed by blowdown. This method is limited to boilers, where feed water is low in hardness salts, to low pressures- high TDS content in boiler water is tolerated, and when only small quantity of water is required to be treated. If these conditions are not applied, then high rates of blowdown are required to dispose off the sludge. They become uneconomical from heat and water loss consideration.
Different waters require different chemicals. Sodium carbonate, sodium aluminate, sodium phosphate, sodium sulphite and compounds of vegetable or inorganic origin are all used for this purpose. Proprietary chemicals are available to suit various water conditions. The specialist must be consulted to determine the most suitable chemicals to use in each case. Internal treatment alone is not recommended.
External Water Treatment
External treatment is used to remove suspended solids, dissolved solids (particularly the calcium and magnesium ions which are a major cause of scale formation) and dissolved gases (oxygen and carbon dioxide).
The external treatment processes available are: ion exchange; demineralization; reverse osmosis and de-aeration. Before any of these are used, it is necessary to remove suspended solids and colour from the raw water, because these may foul the resins used in the subsequent treatment sections.
Methods of pre-treatment include simple sedimentation in settling tanks or settling in clarifiers with aid of coagulants and flocculants. Pressure sand filters, with spray aeration to remove carbon dioxide and iron, may be used to remove metal salts from bore well water.
The first stage of treatment is to remove hardness salt and possibly non-hardness salts. Removal of only hardness salts is called softening, while total removal of salts from solution is called demineralization. The processes are:
Ion-exchange process (Softener Plant)
Softening reaction:
Na2R + Ca(HCO3)2 ‹‹ CaR + 2 Na(HCO3)
Regeneration reaction
CaR + 2 NaCl ‹‹ 2R + CaCl2
In ion-exchange process, the hardness is removed as the water passes through bed of natural zeolite or synthetic resin and without the formation of any precipitate. The simplest type is 'base exchange' in which calcium and magnesium ions are exchanged for sodium ions. After saturation regeneration is done with sodium chloride. The sodium salts being soluble, do not form scales in boilers. Since base exchanger only replaces the calcium and magnesium with sodium, it does not reduce the TDS content, and blowdown quantity. It also does not reduce the alkalinity.
Demineralization is the complete removal of all salts. This is achieved by using a "cation" resin, which exchanges the cations in the raw water with hydrogen ions, producing hydrochloric, sulphuric and carbonic acid. Carbonic acid is removed in degassing tower in which air is blown through the acid water. Following this, the water passes through an "anion" resin which exchanges anions with the mineral acid (e.g. sulphuric acid) and forms water. Regeneration of cations and anions is necessary at intervals using, typically, mineral acid and caustic soda respectively. The complete removal of silica can be achieved by correct choice of anion resin.
Ion exchange processes can be used for almost total demineralization if required, as is the case in large electric power plant boilers
De-aeration
In de-aeration, dissolved gases, such as oxygen and carbon dioxide, are expelled by preheating the feed water before it enters the boiler.
All natural waters contain dissolved gases in solution. Certain gases, such as carbon dioxide and oxygen, greatly increase corrosion. When heated in boiler systems, carbon dioxide (CO2) and oxygen (O2) are released as gases and combine with water (H2O) to form carbonic acid, (H2CO3).
Removal of oxygen, carbon dioxide and other non-condensable gases from boiler feedwater is vital to boiler equipment longevity as well as safety of operation. Carbonic acid corrodes metal reducing the life of equipment and piping. It also dissolves iron (Fe) which when returned to the boiler precipitates and causes scaling on the boiler and tubes. This scale not only contributes to reducing the life of the equipment but also increases the amount of energy needed to achieve heat transfer.
De-aeration can be done by mechanical de-aeration, by chemical de-deration or by both together.
Mechanical de-aeration
Mechanical de-aeration for the removal of these dissolved gases is typically utilized prior to the addition of chemical oxygen scavengers. Mechanical de-aeration is based on Charles' and Henry's laws of physics. Simplified, these laws state that removal of oxygen and carbon dioxide can be accomplished by heating the boiler feed water, which reduces the concentration of oxygen and carbon dioxide in the atmosphere surrounding the feed water. Mechanical de-aeration can be the most economical. They operate at the boiling point of water at the pressure in the deaerator. They can be of vacuum or pressure type.
The vacuum type of de-aerator operates below atmospheric pressure, at about 82oC, can reduce the oxygen content in water to less than 0.02 mg/litre. Vacuum pumps or steam ejectors are required to maintain the vacuum.
The pressure-type de-aerators operates by allowing steam into the feed water through a pressure control valve to maintain the desired operating pressure, and hence temperature at a minimum of 105oC. The steam raises the water temperature causing the release of O2 and CO2 gases that are then vented from the system. This type can reduce the oxygen content to 0.005 mg/litre.
Where excess low-pressure steam is available, the operating pressure can be selected to make use of this steam and hence improve fuel economy. In boiler systems, steam is preferred for de-aeration because:
- - Steam is essentially free from O2 and CO2,
- - Steam is readily available
- - Steam adds the heat required to complete the reaction.
Chemical de-Aeration
While the most efficient mechanical deaerators reduce oxygen to very low levels (0.005 mg/litre), even trace amounts of oxygen may cause corrosion damage to a system. Consequently, good operating practice requires removal of that trace oxygen with a chemical oxygen scavenger such as sodium sulfite or hydrazine. Sodium sulphite reacts with oxygen to form sodium sulphate, which increases the TDS in the boiler water and hence increases the blowdown requirements and make-up water quality. Hydrazine reacts with oxygen to form nitrogen and water. It is invariably used in high pressures boilers when low boiler water solids are necessary, as it does not increase the TDS of the boiler water.
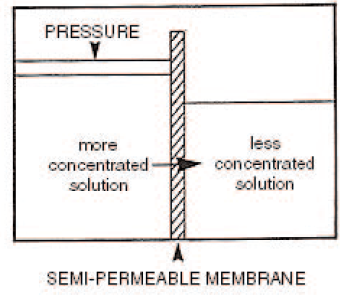
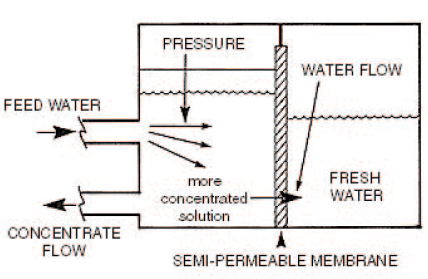
Reverse Osmosis
Reverse osmosis uses the fact that when solutions of differing concentrations are separated by a semi-permeable membrane, water from less concentrated solution passes through the membrane to dilute the liquid of high concentration. If the solution of high concentration is pressurized, the process is reversed and the water from the solution of high concentration flows to the weaker solution. This is known as reverse osmosis. The quality of water produced depends upon the concentration of the solution on the high-pressure side and pressure differential ascross the membrane. This process is suitable for waters with very high TDS, such as sea water.
Recommended boiler and feed water quality
The impurities found in boiler water depend on the untreated feed water quality, the treatment process used and the boiler operating procedures. As a general rule, the higher the boiler operating pressure, the greater will be the sensitivity to impurities. Recommended feed water and boiler water limits are shown in Table 2.2 and Table 2.3.
| TABLE 2.2 RECOMMENDED FEED WATER LIMITS | |||
| Factor | Upto 20 kg/cm2 | 21 - 39 kg/cm2 | 41 - 59 kg/cm2 |
| Total iron (max) ppm | 0.05 | 0.02 | 0.01 |
| Total copper (max) ppm | 0.01 | 0.01 | 0.01 |
| Total silica (max) ppm | 1.0 | 0.3 | 0.1 |
| Oxygen (max) ppm | 0.02 | 0.02 | 0.01 |
| Hydrazine residual ppm | - | - | -0.02-0.04 |
| pH at 25oC | 8.8-9.2 | 8.8-9.2 | 8.2-9.2 |
| Hardness, ppm | 10 | 0.5 | - |
| TABLE 2.3 RECOMMENDED BOILER WATER LIMITS (IS 10392, YEAR 1982) | |||
| Factor | Upto 20 kg/cm2 | 21 - 39 kg/cm2 | 40 - 59 kg/cm2 |
| TDS, ppm | 3000-3500 | 1500-2500 | 500-1500 |
| Total iron dissolved solids ppm | 500 | 200 | 150 |
| Specific electrical conductivity at 25oC (mho) | 1000 | 400 | 300 |
| Phosphate residual ppm | 20-40 | 20-40 | 15-25 |
| pH at 25oC | 10-10.5 | 10-10.5 | 9.8-10.2 |
| Silica (max) ppm | 25 | 15 | 10 |
2.7 Energy Conservation Opportunities
The various energy efficiency opportunities in boiler system can be related to combustion, heat transfer, avoidable losses, high auxiliary power consumption, water quality and blowdown.
Examining the following factors can indicate if a boiler is being run to maximize its efficiency:
1. Stack Temperature
The stack temperature should be as low as possible. However, it should not be so low that water vapor in the exhaust condenses on the stack walls. This is important in fuels containing signficant sulphur as low temperature can lead to sulphur dew point corrosion. Stack temperatures greater than 200oC indicates potential for recovery of waste heat. It also indicate the scaling of heat transfer/recovery equipment and hence the urgency of taking an early shut down for water / flue side cleaning.
2. Feed Water Preheating using Economiser
Typically, the flue gases leaving a modern 3-pass shell boiler are at temperatures of 200 to 300 oC. Thus, there is a potential to recover heat from these gases. The flue gas exit tempera ture from a boiler is usually maintained at a minimum of 200oC, so that the sulphur oxides in the flue gas do not condense and cause corrosion in heat transfer surfaces. When a clean fuel such as natural gas, LPG or gas oil is used, the economy of heat recovery must be worked out, as the flue gas temperature may be well below 200oC.
The potential for energy saving depends on the type of boiler installed and the fuel used. For a typically older model shell boiler, with a flue gas exit temperature of 260oC, an economizer could be used to reduce it to 200oC, increasing the feed water temperature by 15oC. Increase in overall thermal efficiency would be in the order of 3%. For a modern 3-pass shell boiler firing natural gas with a flue gas exit temperature of 140oC a condensing economizer would reduce the exit temperature to 65oC increasing thermal efficiency by 5%.
3. Combustion Air Preheat
Combustion air preheating is an alternative to feedwater heating. In order to improve thermal efficiency by 1%, the combustion air temperature must be raised by 20oC. Most gas and oil burners used in a boiler plant are not designed for high air preheat temperatures.
Modern burners can withstand much higher combustion air preheat, so it is possible to consider such units as heat exchangers in the exit flue as an alternative to an economizer, when either space or a high feed water return temperature make it viable.
4. Incomplete Combustion
Incomplete combustion can arise from a shortage of air or surplus of fuel or poor distribution of fuel. It is usually obvious from the colour or smoke, and must be corrected immediately.
In the case of oil and gas fired systems, CO or smoke (for oil fired systems only) with normal or high excess air indicates burner system problems. A more frequent cause of incomplete combustion is the poor mixing of fuel and air at the burner. Poor oil fires can result from improper viscosity, worn tips, carbonization on tips and deterioration of diffusers or spinner plates.
With coal firing, unburned carbon can comprise a big loss. It occurs as grit carry-over or carbon-in-ash and may amount to more than 2% of the heat supplied to the boiler. Non uniform fuel size could be one of the reasons for incomplete combustion. In chain grate stokers, large lumps will not burn out completely, while small pieces and fines may block the air passage, thus causing poor air distribution. In sprinkler stokers, stoker grate condition, fuel distributors, wind box air regulation and over-fire systems can affect carbon loss. Increase in the fines in pulverized coal also increases carbon loss.
5. Excess Air Control
The Table 2.4 gives the theoretical amount of air required for combustion of various types of fuel.
Excess air is required in all practical cases to ensure complete combustion, to allow for the normal variations in combustion and to ensure satisfactory stack conditions for some fuels. The optimum excess air level for maximum boiler efficiency occurs when the sum of the losses due to incomplete combustion and loss due to heat in flue gases is minimum. This level varies with furnace design, type of burner, fuel and process variables. It can be determined by conducting tests with different air fuel ratios.
| TABLE 2.4 THEORETICAL COMBUSTION DATA - COMMON BOILER FUELS | |||||
| Fuel | kg of air req./kg of fuel | kg of flue gas/kg of fuel | m3 of flue/kg of fuel | Theoretical CO2% in dry flue gas | CO2% in flue gas achieved in practice |
| Solid Fuels Bagasse Coal (bituminous) Lignite Paddy Husk Wood | 3.2 10.8 8.4 4.6 5.8 | 3.43 11.7 9.10 5.63 6.4 | 2.61 9.40 6.97 4.58 4.79 | 20.65 18.70 19.40 19.8 20.3 | 10-12 13-13 9-13 14-15 11.13 |
| Liquid Fuels Furnace Oil LSHS | 13.90 14.04 | 14.30 14.63 | 11.50 10.79 | 15.0 15.5 | 9-14 9-14 |
Typical values of excess air supplied for various fuels are given in Table - 2.5.
| TABLE 2.5 EXCESS AIR LEVELS FOR DIFFERENT FUELS | ||
| Fuel | Type of Furnace or Burners | Excess Air (% by wt) |
| Pulverised coal | Completely water-cooled furnace for slag-tap or dry-ash removal | 15-20 |
| Partially water-cooled furnace for dry-ash removal | 15-40 | |
| Coal | Spreader stoker | 30-60 |
| Water-cooler vibrating-grate stokers | 30-60 | |
| Chain-grate and traveling-gate stokers | 15-50 | |
| Underfeed stoker | 20-50 | |
| Fuel oil | Oil burners, register type | 15-20 |
| Multi-fuel burners and flat-flame | 20-30 | |
| Natural gas | High pressure burner | 5-7 |
| Wood | Dutch over (10-23% through grates) and Hofft type | 20-25 |
| Bagasse | All furnaces | 25-35 |
| Black liquor | Recovery furnaces for draft and soda-pulping processes | 30-40 |
Controlling excess air to an optimum level always results in reduction in flue gas losses; for every 1% reduction in excess air there is approximately 0.6% rise in efficiency.
Various methods are available to control the excess air:
- Portable oxygen analysers and draft gauges can be used to make periodic readings to guide the operator to manually adjust the flow of air for optimum operation. Excess air reduction up to 20% is feasible.
- The most common method is the continuous oxygen analyzer with a local readout mounted draft gauge, by which the operator can adjust air flow. A further reduction of 10-15% can be achieved over the previous system.
- The same continuous oxygen analyzer can have a remote controlled pneumatic damper positioner, by which the readouts are available in a control room. This enables an operator to remotely control a number of firing systems simultaneously.
The most sophisticated system is the automatic stack damper control, whose cost is really justified only for large systems.
6. Radiation and Convection Heat Loss
The external surfaces of a shell boiler are hotter than the surroundings. The surfaces thus lose heat to the surroundings depending on the surface area and the difference in temperature between the surface and the surroundings.
The heat loss from the boiler shell is normally a fixed energy loss, irrespective of the boiler output. With modern boiler designs, this may represent only 1.5% on the gross calorific value at full rating, but will increase to around 6%, if the boiler operates at only 25 percent output.
Repairing or augmenting insulation can reduce heat loss through boiler walls and piping.
7. Automatic Blowdown Control
Uncontrolled continuous blowdown is very wasteful. Automatic blowdown controls can be installed that sense and respond to boiler water conductivity and pH. A 10% blow down in a 15 kg/cm2 boiler results in 3% efficiency loss.
8. Reduction of Scaling and Soot Losses
In oil and coal-fired boilers, soot buildup on tubes acts as an insulator against heat transfer. Any such deposits should be removed on a regular basis. Elevated stack temperatures may indicate excessive soot buildup. Also same result will occur due to scaling on the water side.
High exit gas temperatures at normal excess air indicate poor heat transfer performance. This condition can result from a gradual build-up of gas-side or waterside deposits. Waterside deposits require a review of water treatment procedures and tube cleaning to remove deposits. An estimated 1% efficiency loss occurs with every 22oC increase in stack temperature.
Stack temperature should be checked and recorded regularly as an indicator of soot deposits. When the flue gas temperature rises about 20oC above the temperature for a newly cleaned boiler, it is time to remove the soot deposits. It is, therefore, recommended to install a dial type thermometer at the base of the stack to monitor the exhaust flue gas temperature.
It is estimated that 3 mm of soot can cause an increase in fuel consumption by 2.5% due to increased flue gas temperatures. Periodic off-line cleaning of radiant furnace surfaces, boiler tube banks, economizers and air heaters may be necessary to remove stubborn deposits.
9. Reduction of Boiler Steam Pressure
This is an effective means of reducing fuel consumption, if permissible, by as much as 1 to 2%. Lower steam pressure gives a lower saturated steam temperature and without stack heat recovery, a similar reduction in the temperature of the flue gas temperature results.
Steam is generated at pressures normally dictated by the highest pressure / temperature requirements for a particular process. In some cases, the process does not operate all the time, and there are periods when the boiler pressure could be reduced. The energy manager should consider pressure reduction carefully, before recommending it. Adverse effects, such as an increase in water carryover from the boiler owing to pressure reduction, may negate any potential saving. Pressure should be reduced in stages, and no more than a 20 percent reduction should be considered.
10. Variable Speed Control for Fans, Blowers and Pumps
Variable speed control is an important means of achieving energy savings. Generally, combustion air control is effected by throttling dampers fitted at forced and induced draft fans. Though dampers are simple means of control, they lack accuracy, giving poor control characteristics at the top and bottom of the operating range. In general, if the load characteristic of the boiler is variable, the possibility of replacing the dampers by a VSD should be evaluated.
11. Effect of Boiler Loading on Efficiency
The maximum efficiency of the boiler does not occur at full load, but at about two-thirds of the full load. If the load on the boiler decreases further, efficiency also tends to decrease. At zero output, the efficiency of the boiler is zero, and any fuel fired is used only to supply the losses. The factors affecting boiler efficiency are:
- As the load falls, so does the value of the mass flow rate of the flue gases through the tubes. This reduction in flow rate for the same heat transfer area, reduced the exit flue gas temperatures by a small extent, reducing the sensible heat loss.
- Below half load, most combustion appliances need more excess air to burn the fuel completely. This increases the sensible heat loss
In general, efficiency of the boiler reduces significantly below 25% of the rated load and as far as possible, operation of boilers below this level should be avoided
12. Proper Boiler Scheduling
Since, the optimum efficiency of boilers occurs at 65-85% of full load, it is usually more efficient, on the whole, to operate a fewer number of boilers at higher loads, than to operate a large number at low loads.
13. Boiler Replacement
The potential savings from replacing a boiler depend on the anticipated change in overall efficiency. A change in a boiler can be financially attractive if the existing boiler is :
- old and inefficient
- not capable of firing cheaper substitution fuel
- over or under-sized for present requirements
- not designed for ideal loading conditions
The feasibility study should examine all implications of long-term fuel availability and company growth plans. All financial and engineering factors should be considered. Since boiler plants traditionally have a useful life of well over 25 years, replacement must be carefully studied.
2.8 Case Study
Installing Boiler Economiser
A paper mill retrofitted an economiser to existing boiler. The general specification of the boiler is given below:
| Boiler Capacity (T/h) | Feed Water Temp (oC) | Steam Pressure (bar) | Fuel oil |
| 8 | 110 | 18 | Furnace oil |
The thermal efficiency of the boiler was measured and calculated by the indirect method using flue gases analyser and data logger. The result is summarised below:
| Thermal efficiency | : 81% |
| Flue gas temperature | : 315oC |
| CO2% | : 13 |
| CO (ppm) | : 167 |
The temperature in the flue gas is in the range of 315 to 320oC. The waste heat in the flue gas is recovered by installing an economizer, which transfers waste heat from the flue gases to the boiler feed water. This resulted in a rise in feed water temperature by about 26oC.
Basic Data
| • | Average quantity of steam generated ..... | : 5 T/hr |
| • | Average flue gas temperature ................ | : 315oC |
| • | Average steam generation / kg of fuel oil | : 14 kg |
| • | Feed water inlet temperature ................. | : 110oC |
| • | Fuel oil supply rate................................ | : 314 kg/hr |
| • | Flue gas quantity ................................... | : 17.4 kg/kg of fuel |
Cost Economics
| • | Quantity of flue gases ............................. | : 314 × 17.4 = 5463.6 kg/h |
| • | Quantity of heat available in the flue gases | : 5463.6 × 0.23 × (315-200) = 144512 kCal/h |
| • | Rise in the feed water temperature ........... | : 26oC. |
| • | Heat required for pre-heating the feed water | : 5000 × 1 × 26 = 130000 kCal/h |
| • | Saving in terms of furnace oil ..................... | : 130000/10000 = 13 kg/h |
| • | Annual operating hours ........................... | : 8600 |
| • | Annual savings of fuel oil ......................... | : 8600 × 13 = 111800 kg |
Conclusion
Through recovery of waste heat by installation of an economizer, the paper mill was able to save 13 kg/hr. of furnace oil, which amounts to about 1,11,800 kg of furnace oil per annum.
| QUESTIONS | |
| 1. | What is the importance of draft in boilers? |
| 2. | What is a balanced draft system? |
| 3. | Which is the single major heat loss in boiler? |
| 4. | Explain the principle of modulating control in a boiler? |
| 5. | Explain the principle of fire tube and water tube boilers? |
| 6. | Explain the principles of fluidized bed combustion and pulverized fuel combustion? |
| 7. | Name three factors affecting the boiler efficiency and explain briefly? |
| 8. | Discuss the various types of draft in boiler system? |
| 9. | What do you understand by terminology fire tube and water tube in boiler? |
| 10. | Discuss the various types of heat losses in a boiler? |
| 11. | How do you measure boiler efficiency using direct method? |
| 12. | What do you understand by term evaporation ratio? What are the typical values for coal and oil-fired boiler? |
| 13. | What do you understand by the term 'Turn Down Ratio' ? |
| 14. | What are the methods available for assessing the boiler efficiency and explain briefly? |
| 15. | How do you assess boiler blow down requirement? |
| 16. | Discuss automatic blow down control system? |
| 17. | Why blow down is given in boiler? |
| 18. | What is the function of de-aerator in boiler? |
| 19. | What is the difference between an economizer and an air pre heater? |
| 20. | List the 5 energy conservation measures in improving the boiler efficiency without investment. |
| 21. | What is intermittent and continuous blow down? |
| 22. | Why is sulphur in coal undesirable? |
| 23. | Is moisture in coal wasteful? |
| 24. | What is atomisation of fuel oil in combustion? |
| 25. | What are the causes for heavy black smoke in a boiler? |
| 26. | 1 kg of water at 25oC is converted in to steam at atmospheric condition. What is the value of sensible heat and latent heat added to the steam? |
| 27. | For boiler at 8 kg/cm2 (g) steam pressure. The following details are given Saturation temperature of steam = 170oC Sensible heat of water = 171 kCal/kg Latent heat of evaporation = 490 kCal/kg Moisture content in the steam = 4% What is the total heat content of the steam? |
| 28. | The following are the ultimate analysis for coal: Calculate the stoichiometric air requirement. Carbon-38%, Ash-35%, Hydrogen-5% , Sulphur-2%. For the same data, calculate the theoretical CO2. If the actual measured CO2 is 8%, find out the excess air levels? |
| 29. | A packaged boiler is operating at 5% O2. Find out the excess air level? |
| 30. | In a furnace oil fired boiler, the evaporation ratio (kg of steam generated / kg of furnace oil) was found to be 20 against a best possible limit of 13. (a) in your opinion what could be the reasons for the same? (b) would you like to recommend the user to maintain the same practice and conditions as the evaporation ratio is more than the feasible limit? |
REFERENCES
- Steam Boiler Room Questions & Answers, Third Edition by Stephen M.Elonka and Alex Higgins
- Steam Boiler Operation by James J.Jackson, Prentice-Hall Inc, New Jersey, 1980.
- Boilers by Carl D. Shields, McGraw Hill Book Company, U.S, 1961.
- Industrial Heat Generation and Distribution -NIFES Training Manual Issued For CEC - India Energy Bus Project
- Practical Boiler Water Treatment by Leo.I.Pincus, McGraw Hill Inc, New York, 1962.
- Technical Papers, Boiler Congress-2000 Seminar, 11 & 12 January 2000
- Industrial Boilers by David Gunn and Robert Horton, Longman Scientific & Technical, New York
- Steam Generation, Distribution and Utilisation by TERI, GTZ and EMC
- Efficient Operation of Boilers by National Productivity Council