Gases & Liquids (Fluids) and Solids:

The compound H2O (2 molecules of the element Hydrogen combined with 1 molecule of the element Oxygen) can exist in 3 states. They are solid (ice), liquid (water) and gas (steam). This is theoretically true of all elements and compounds. Liquids and gases can be grouped together as Fluids. Fluids have common performance characteristics. For example, the flow of air in ducts and the flow of water in pipes are based on the same theory and equations. The study of HVAC requires understanding gases (particularly Air) and liquids (particularly Water).
The term "Fluid" consists of gases and liquids. Fluids have a definite mass and volume at a given temperature and pressure. They have no consistent shape when it is not confined a container. They cannot sustain shear (lateral) stress under equilibrium conditions. They cannot be torn, fractured or broken into smaller pieces.
Gases are fluids that do not have a definite volume. A gas has no shape and it assumes the volume of the container that it is confined in. Gases can be compressed. They are affected by temperature and pressure. The volume of gas in an enclosed container is the volume of the container. Two containers of different volumes can contain the same mass of gas.
Vapors are gases that can condense at everyday atmospheric pressures and temperatures whereas dry gases condense at very high pressures and very low temperatures. For example, water boils at 212oF (100oC) at standard atmospheric pressure of 14.7 pounds per square inch absolute (psia), and becomes steam at 212oF. However, the moisture content of air is water vapor (steam) at a temperature of, say, 70oF. This is explained later with Dalton's law of partial pressures.
Liquids are fluids that have a definite volume of their own that is independent of the shape and volume of the container. When a liquid is placed in a container, it assumes the shape of the container but the volume and mass remain the same under constant temperature and pressure. Liquids can be considered non-compressible. The volume will not change significantly under pressure. The volume of the liquid can change appreciably at different temperatures. This property is used in the mercury thermometer.
A liquid exposed to atmospheric pressure will have a free-standing surface. All the points of the top surface of the liquid are at the same level. For example, "sea-level" is the common level of all the seas and oceans that are connected. Land levels or "altitudes" are measured from this level.

Figure
There is an explanation for why the Atlantic and Pacific Oceans are at slightly different levels and "locks" have to be used to move ships up and down through the Panama Canal.
Solids: The strong bond between the molecules in a solid makes it very rigid and difficult to deform. Solids tend to maintain their volume and shape, but this can vary slightly with tensional (lateral) and torsional (twisting) stresses (pressures) and temperatures.
Mass & Weight
The mass (m) of an object is a fundamental property of the object; a numerical measure of its inertia; a fundamental measure of the amount of matter in the object. All mechanical quantities can be defined in terms of mass, length and time. Definition of mass and measuring mass is usually a circular process. Theoretically all masses have to be measured relative to a standard mass or against a known mass using a balance.
The weight (w) of an object is the force of gravity on the mass. Weight can be measured by placing the object on a scale. It is defined as the mass times the acceleration due to gravity (g). W = m*g. The mass of an object has a volume (v) which means that all masses (including gases such as air) occupies space
The density (d) of an object is mass / volume (m/v).
The mass of an object does not change. The weight changes with location. For example, a person with a weight of 200 pounds on earth will have a weight on the moon which is less than a third of that on the earth because the gravitational force is smaller. In outer space the person will have no weight. The person weighs more when he is going up in an elevator and less when going down. The mass of the person remains at 200 pounds at any location in the universe. Similarly, the density and volume of the object does not change.
The terms mass and weight have been used loosely in every day language to mean the same thing. That is because if you remained on the planet earth it does not matter. For example the term pound is actually a weight which is the force of gravity on a mass. Mass, in English units is the poundal, a term that is rarely used. The term kilogram in metric units refers to mass and the metric weight (force) is called the Newton. Yet we have the conversion factor 1 kg = 2.2 lbs.
When the terms "pound" or "kilogram" is used in heat calculations it is referring to mass, and when it is used in pressure or force calculations it is referring to weight.
Properties of Solids, Liquids & Gases

Temperature
Temperature is not heat and temperature by itself is not a measure of the quantity of heat. It is a measure of heat intensity. Temperature is a measure of the human sense of feeling for hot and cold. It is a quality just like good, bad, tall and short but it has a numeric scale. The sense of touch can be a misleading measure since steel at 150oF will feel much hotter than wood at the same temperature since heat is conducted much faster from steel. Technically, temperature is an expression of the molecular excitation of a substance. Like length and weight, the measure of hot and cold were subjectively created by people in different ways in different parts of the world. Water was used to develop the hot and cold temperature measuring scale.
In the S-I Celsius scale the freezing point of water was set as 0oC and the boiling point of water was set as 100oC and there are 100 Celsius degrees between freezing and boiling. The points were set at standard atmospheric pressure conditions. In the I-P Fahrenheit scale the freezing point of water was set as 32oF and the boiling point of water was set as 212oF and there are 180 Fahrenheit degrees between freezing and boiling. The relationship Celsius and Fahrenheit is: oC = ( oF - 32 ) * 5 / 9 and oF = 32 + oC * 9 / 5.
Absolute Temperature (T):
The relationship between the volume and temperature of a gas is linear. The volume of a gas

Figure -
If the lines representing the linear relationship between gas temperature and gas volume, for any element or compound in the gaseous state, then at a certain low temperature the theoretical volume of the gas will become zero. The temperature at which the theoretical volume of zero is reached is the same for all gases. This temperature is -460oF in the Fahrenheit scale and -273oC in the Celsius scale. This temperature is called the absolute zero temperature and temperatures measured from this absolute zero are called absolute temperatures. S-I units are degrees Kelvin (oK) and I-P units are degrees Rankin (oR).
Charles Law (Gases)
The Volume (V) of a given mass (M) of a gas is directly proportional to the Absolute Temperature (T) at constant Pressure (P)
V < T or V / T = Constant or V1 / T1 = V2 / T2 = V3 / T3 = ------ = Vn / Tn
Where T = absolute temperature = ToR ( 460 + toF ) or ToK ( 273 + toC )
Pressure (P):
Pressure is the force per unit area. The pressure exerted by a force or weight of 100 lbs on 10 ft2 is (100 lbs / 10 ft2) 10 pound per square foot (1 psf). The same weight resting on 40 ft2 is (100 lbs / 40 ft2) is 2.5 pounds per square foot (2.5 psf) or 2.5 lbs on 144 square inches = 0.01736 pounds per square inches (psi).

Figure -
Solids and liquids can be considered incompressible. So the volume of the solid or liquid shown in Figure-? does not change with shape. Gases are compressible. So a given mass of gas can occupy different volumes at different pressures.

Boyle's Law (Gases)
The volume (V) of a given mass (M) of gas at a constant temperature (T) is inversely proportional to the pressure (P)
V < 1 / T or PV = Constant or P1V1 = P2V2 = P3V3 = ---- = PnVn at T = Constant
Where T = absolute temperature = ToR ( 460 + toF ) or ToK ( 273 + toC )
The General Gas Law
Charles' Law and Boyle's Law can be combined to get
PV/T = Constant or P1V1/T1 = P2/V2/T2 = P3V3/T3 = ---- = PnVn/Tn
Since the mass of gas (m) is the same when applying Charles and Boyles laws, the general gas law is expressed as
PV = MRT R = Ro / Mw
- Where (I-P units)
- P = Pressure of the gas (lbs/ft3)
- V = Volume of the gas (ft3)
- T = Absolute Temperature ( oR = oF+460)
- M = Mass of Gas (lbs)
- R = Gas Constant (Constant of Proportionality)
- Ro = Universal Gas Constant = 1545.4
- Mw = Molecular weight of the gas
For example the molecular weight of steam (H2O) is the atomic weights of it's chemical elements which is 2 atoms of hydrogen at 1.01 combined with 1 atom of oxygen at 16 = 18.02. So R for steam is = 1545.4/18.02 = 85.78 ft-lbf/lboR
Density (d) is the mass per unit volume. Example: Density of water is approximately 62.4 pounds per cubic foot (I-P) and 1000 kilograms per cubic meter (S-I) at normal pressure and temperature.

Figure -
Specific Gravity (Sg)
Specific Gravity (Sg) is the relative density of substances with respect to water. For example, the density of a 20% solution of calcium chloride (CaCl2) is 73.8 lbs/ft3. Since the density of water is 62.4 lbs/ft3, the specific gravity of CaCl2 is (73.8/62.4) 1.18 or it is 1.18 times heavier than water. The density of kerosene oil is 51.2 lbs/ft3 and so it's specific gravity (51.2/62.4) is 0.69. Kerosene is lighter than water. So the specific gravity of a substance indicates how much heavier ( > 1) or lighter ( < 1 ) it is than water ( = 1 )
Specific Volume (Vs)
Specific Volume (Vs) is the volume per unit mass. It is the reciprocal of density. Since the density of water is 62.4 lbs/ft3 or 1000 kgs/m3, the specific volume is 0.016 ft3 / lb or 0.001 m3 / kg.
Heat (Q)
Heat is a form of energy just like nuclear, chemical, potential, kinetic and electrical energy. Energy can be converted from one form to another. Heat is a quantity just like volume and mass. Quantities can be added and subtracted unlike qualities like temperature. For example 10 lbs of water at 100oF when added to 10 lbs of water at 50oF will not produce 20 lbs of water at 150oF. However 100 heat units can be added to 50 heat units to get 150 heat units. The quantity of heat in a substance depends on the mass (lbs) of the substance and it's temperature (oF).
As with distances measured in feet or meters and weights measured in pounds or kilograms, heat is measured from a traditionally chosen reference point and the measuring scale is set arbitrarily.
In I-P units a single unit of heat is the energy required to raise one pound of pure water through one degree Fahrenheit. The amount of heat required to raise 1 lb of water from 33oF to 34oF is more than the heat required to raise 1 lb of water from 210oF to 211oF. So more specifically the unit of heat in I-P units is the amount of heat required to raise 1 lb of water from 70oF to 71oF. This is called the British Thermal Unit (BTU).
In S-I units, the unit measure of heat is the heat required to raise one gram of pure water through 1 oC or more specifically from 14.5 oC to 15.5 oC. This unit is called the Calorie.
Example: The amount of heat required to raise 15 lbs of water from 50 oF to 140 oF is = 15 lbs x ( 140oF - 50oF ) = 1350 btu. The equation for water is mass (lbs) x difference in temperature (oF) or h = m *(t2 -t1).
Specific Heat (Sh)
Specific Heat (Sh) of a substance is the amount of heat required to raise 1 lb of the substance through 1oF. Since it requires 1 btu to raise 1 lb of water through 1 oF the specific heat of water is equal to 1. Like Specific Gravity, Specific Heat can therefore also be defined as the ratio ( < 1, = 1, > 1 ) of the heat required to raise the mass of substance through a particular temperature rise to the amount of heat required to raise the same mass of water through the same temperature rise.
Sh = (Heat required by unit mass M of substance to raise it's temperature unit temp rise
Q = M * Sh * (t2 - t1) or Q = M * Sh * (T2 - T1) where
- T1 (oR, oK) or t1 (oF, oC) = initial temperature of substance
- T2 (oR, oK) or t2 (oF, oC) = final temperature of substance
- Q = heat gain ( T2, t2 > T1, t1) or loss ( T2, t2 < T1, t1) of the substance
- Sh = Specific Heat (btu/lb.oF , (J/kg.oK)
Alternatively specific heat can be defined as the heat required to raise a unit mass of the substance through one degree. Example the heat required to raise 1 lb of water through 1oF is 1 btu so the specific heat of water is 1. This is by definition. The heat required to raise 1 lb of aluminum through 1oF is 0.22 btu. So the specific heat of aluminum is 0.22. Since specific heat is the ratio of two like units it is a number without units.
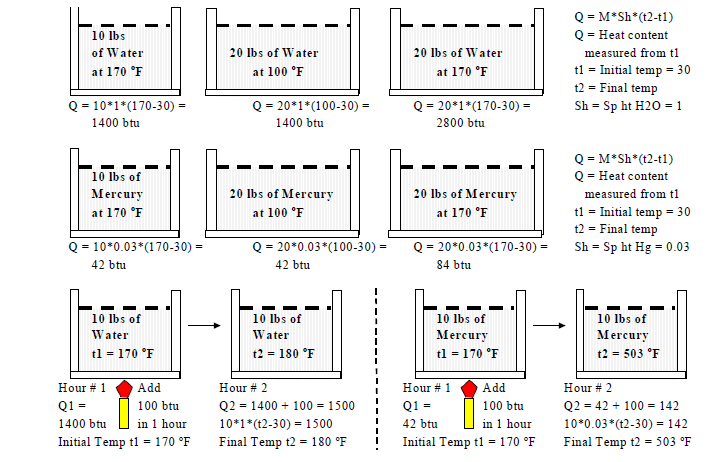
Figure-
Specific heat can vary with temperature. For example the heat required to raise 1 lb of water fro 33oF to 34oF is slightly more than the heat required to raise 1 lb of water from 210oF to 211oF. For most liquids and solids this variation can be neglected but it cannot be neglected in the case of gases. The specific heat of gases measured at constant volume (symbol Cv) and constant pressure (symbol Cp) are different. Since most processes in air conditioning occur at atmospheric pressure, the default specific that is used is that at constant pressure (Cp).
Measuring Temperature
Solids, liquids and gases expand with increasing temperature and contract with decreasing temperature. This property is used to measure temperature. The amount of change varies with different substances and different liquids and solids are used to measure temperature for different temperature ranges and for different conditions.
A common liquid used to measure atmospheric temperature is Mercury. It consists of a glass capillary tube filled with Hg. The tube has a bulb at the bottom (to provide greater surface contact with air) and the top is a vacuum space. Alcohol has a higher coefficient of expansion than Mercury (it expands much more for a small change in temperature) but it is unsuitable for measuring air temperatures. Air temperatures can vary from -40oF to 130oF (-40oC to 55o). The length of a mercury thermometer for this temperature range is about 1 foot (0.3 meters).
The disadvantages of the mercury thermometer are that it's bulb surface must be surrounded by the substance being measured, it is bulky, it can break easily, and it is unsuitable for very high temperatures. The current flowing through a coil of wire varies with the temperature of the wire and this property is used in the electric resistance type thermometer. Thermocouples are used to measure the temperature at a point. A thermocouple consists of two different types of metals.
Measuring Heat
There is no instrument for measuring the quantity of heat directly. The quantity of heat contained in a substance is the product of the mass, it's specific heat and it's temperature above a selected reference temperature. The heat quantity in a substance is a positive or negative number depending on whether the selected reference temperature point is below or above the temperature of the substance.
For example if the heat quantity of 10 lbs of kerosene with a specific heat of 0.5 is measured from 32oF (the freezing point of water) and the temperature of the kerosene is 12oF, then it contains a negative quantity of heat equal to 10 lbs * 0.5 * (12-32) = -100 btu. In reality there is no such thing as negative heat. All heat quantities would be positive if heat was measured from absolute zero temperature (-273oC or -460oF)
Example: 10 lbs of aluminum with a specific heat of 0.22 was heated from 50oF to 150oF. How much heat was added? Q = M*Sh*(t2-t1) = 10*0.22*(150-50) = 220 btu.
Example: 100 lbs of glycerin at 200oF with a specific heat of 0.58 loses 10000 btu of heat. What is the final temperature of the glycerin? Q = M*Sh*(t2-t1) or 10000 = 100*0.58*(200-t1) or 10000 = 11600 - 58*t1 or t1 = (11600 - 10000) / 58 = 27.6oF.
Example: A liquid weighing 10 lbs is heated from 50oF to 150oF and 300 btu is required to accomplish this. What is the specific heat of the liquid? Q = M*Sh*(t2-t1 or 300 = 10*Sh*(150-50) or 300 = 1000*Sh or Sh = 300 / 1000 = 0.3
Water
Water is a liquid that is odorless, colorless and tasteless. Water at normal air pressures and temperatures is a liquid. A molecule of Water is a compound of two gases – 2 atoms of Hydrogen combined with 1 atom of Oxygen (H2O). Water can be considered to be available free and in abundance. It stores heat, absorbs heat and gives up heat readily.
So besides air, the general medium for distributing heating and cooling energy in the case of large commercial building projects is water. Chilled water generated by refrigeration chillers is used for cooling and water in the form of solid ice is used for storing cooling energy. Hot water and water in the gaseous state of steam is used for heating. Steam is also used by absorption chillers for cooling and by generators for electricity. For small buildings the air can be cooled directly with refrigeration units in summer and heated directly by electricity or fuels such as natural gas heaters in winter. Plumbing and fire protection services are also based on water.
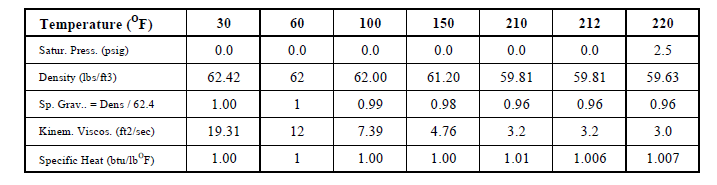
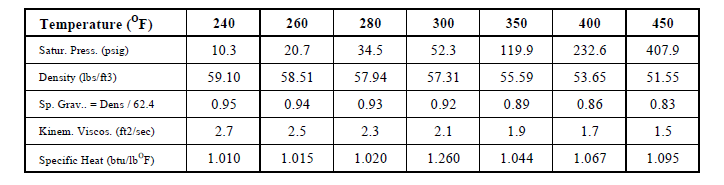
Table -
Pressure Units as Height of Column of Water
Figure ? shows that a measuring scale for pressure can be the height of a column of liquid. For example the density of water is 62.4 lbs/ft3. The pressure at the bottom of a tank with any surface areas and containing water to a height of 1 foot is 62.4 lbs/ft2 and at a height of 4 feet it is 4 times the pressure that of 1 foot. The first tank in the figure contains 500 lbs of water but the pressure at the bottom of the tank of height 2 feet is 2 x 62.4 = 128.4 lbs/ft2. The second tank contains 250 lbs of water but the pressure at the bottom of the tank of height 4 feet is 4 x 62.4 = 249.6 lbs/ft2.
Pressure can therefore be expressed as the height of a column of water. In the case of liquids (water) and pumping systems it is measured in feet of water. In the case of gases (air) and fan systems it is meaured in inches of water.
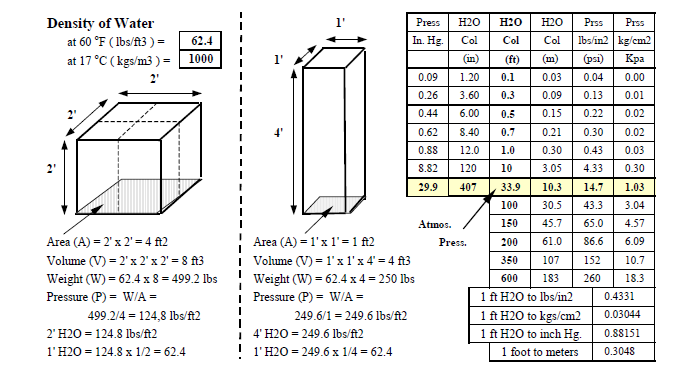
Figure -
Dry Air:
The term "Air" refers to a mixture of gases which can include small quantities of steam (H2O), water that evaporates into the air under various conditions), carbon dioxide (CO2, breathed out by animal life), and artificially added pollutants such as carbon monoxide (CO, emitted by automobiles and other processes. The term "Dry Air" excludes water vapor (H2O) but can include pollutants such as carbon monoxide (CO) and other fumes generating by industrial processes. The composition of Dry Air and it's molecular weight is shown below.

- (28.02*0.7803) + (32.00*0.2099) + (44.00*0.0003) + (2.02*0.0001) + (39.91*0.0094) = 28.97.
- The Gas Constant for Dry Air is = 1545 / 28.97 = 53.34 ft-lbf/lboR
- The Specific Heat of dry air is 0.21 btu/lb.oF.
Although nitrogen and oxygen make up 99% of air, controlling the other 1% is a major part of comfort air-conditioning. People breathe in O2 and breathe out CO2, so CO2 is a measure of the occupancy level of the space and determines the ventilation required to make up the O2. Automobiles and other engines generate CO, so CO is a measure of the cars running in an enclosed garage and determines the ventilation required to dilute and eliminate the CO. Water vapor affects comfort conditions. In winter moisture has to be added to the air in the space and in summer moisture has to be removed from the space.
Atmospheric Air Pressure
The term "air" when referred to in comfort air-conditioning consists of water vapor and dry air (DA) which consists of the rest of gases in the mixture of gases. Steam (water, H2O) is not a mixture of different substances but a stand-alone chemical compound.
The surface of the earth is covered with air. Like all object masses, the mass of air is attracted towards the planet earth by gravity. Traces of air can exist up to 200 miles ( 1 million feet) above the earth. However, 90 percent of the mass of atmospheric air is within 10 miles (53,000 feet, 16,100 meters) of the earth's surface.
This mass of air exerts a pressure on the surface of the earth and this is called atmospheric air pressure. On average this pressure at sea level is approximately 14.7 pounds per square inch or psi ( 34 feet (10.3 meters) of water, 30 inches (750 millimeters ) of mercury, 1 kilogram per square centimeter. Pressure is also measured in atmospheres where 1 atmosphere = 14.7 psi.
The highest point of the earth above sea level is Mount Everest in the Himalayan mountain range separating India and Tibet in China. The height of land measured above sea level is called elevation above sea level or altitude and the altitude of Mt. Everest is 29,029 feet (8,948 meters). The highest capital city of a country is La Paz in Bolivia, South America which is at an altitude of 12,000 feet. Passenger aircraft fly at altitudes of 20,000 to 70,000 feet and this height is still well within the earth's gravitational pull.
The density (lbs/ft3) of air therefore decreases with the elevation above sea level of the location. Since we breathe in the same volume of air at any elevation, the mass amount (lbs) of air, and consequently the amount of oxygen, that we breathe in decreases with increasing elevations. The decrease in density also affects the operation of several types of equipment that use or handle air. These equipment are therefore rated for zero elevation and they have to be reconfigured or reselected for higher elevations.
The pressure of liquids is measured in feet of water and the pressure of gases is measured in inches of water. The pressure of atmospheric air is measured in inches of mercury (in.Hg.).
The density of air also varies with the temperature as shown in the table below. Outdoor air at -20 oF is much denser or heavier than the air at 70oF indoor temperature. Outdoor air in winter is therefore at a higher pressure than the air indoors. This results in the infiltration of outdoor air through the building envelope (cracks around the windows and doors and through porous walls) into the indoor space.

Figure -

Table -
Gauge Pressure
Every surface or object on the earth has the weight of the atmosphere resting on it. The absolute weight or pressure exerted by a substance is therefore the weight of the substance resting on the weighing scale plus the weight of air covering the weighing scale. The sum of these two pressures is called absolute pressure.
The pressure exerted by the atmosphere can be ignored for everyday situations and we can measure pressures and weights above atmospheric pressure. The pressure that excludes atmospheric pressure is called gauge pressure.
The absolute pressure cannot be ignored in scientific calculations since the atmospheric pressure varies with altitude. The absolute units for pressure must be used in dealing with equations such as the Gas Laws.
Absolute Pressure (psia) = Gauge Pressure (psig) + 14.7 psi (atmospheric pressure)
Gauge Pressure (psig) = Absolute Pressure (psia) - 14.7 psi (atmospheric pressure)
It is impossible to make a pressure measurement on the earth's surface unless it is made relative to atmospheric pressure. Pressure gauges, piezometers and all pressure measuring devices indicate gauge pressure, that is pressure above 14.7 psia. Zero psia is supposed to be a perfect vacuum. This cannot be achieved in practice.

Figure -
Measuring Pressure

Figure -
The instrument used to measure atmospheric pressure is called a barometer. Mercury is used to measure this pressure since an instrument using a column of water that is 34 feet high is not practical. The density of mercury is 849.4 lbs/ft3 compared to 62.4 lbs/ft3 of water. The principle of measuring atmospheric pressure is shown in Figure - ?. A tube that is closed at one end is first inverted and filled with the liquid being used to measure pressure. The open end is next held closed and turned over and then immersed in a tank containing the same liquid. The end is then opened. The liquid in the tube will rise until it reaches a height that has the same equivalent pressure of the atmosphere at the level of the tank. The space above the level in the tube is a vacuum at 0 pressure. As with measuring temperature, the mercury barometer is not suitable for measuring very high and low pressures and other types of instruments are used.
Water Vapor (Steam) and Vapor Pressure
Water vapor is a term used to describe the existence of steam in air. Water vapor is a gas that occupies the same space as the other gases that constitute the mixture of gases called air. Although water vapor is one of the gases in the air mixture, in many ways water vapor acts differently and independently of all the other gases in air. This is because water vapor (steam) can condense into a liquid (water) within the earth's range of atmospheric pressures and temperatures, whereas all the other gases in the air mixture will remain a gas
Table-x shows that steam can exist in air and water can exist as water over a very wide range of temperatures depending on the partial pressure of the steam in the air mixture and the pressure under which the water exists. For example, steam at a temperature of 450oF will condense into water at 450oF if the pressure is 422 psia; at 14.7 psia steam will condenses into water 212oF; and at 0 oF steam will condense into water at 0.0186 psia.
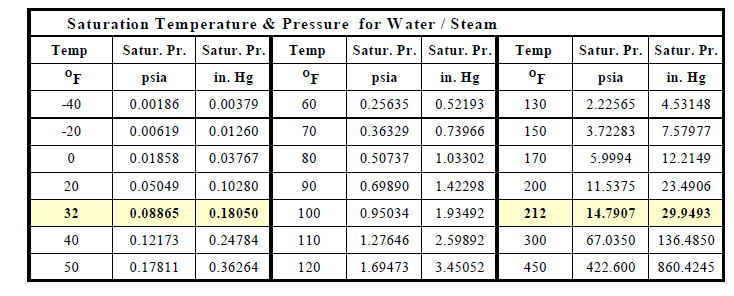
Table -
Dalton's Law of Partial Pressures
Dalton's Law states that the pressure exerted by a mixture of gases (including vapors) is equal to the sum of the pressures of each gas in the mixture. Each gas in the mixture behaves as if it alone occupies the space or volume. Of the standard air pressure of 14.7 psia at 80 oF, only 0.5 psia might be that of the steam and the rest consists of the partial pressures of the other gases in the air such as oxygen and nitrogen.
Air is a mixture of elements and compounds consisting of Nitrogen (N2), Oxygen (O2), Hydrogen (H2), Carbon Dioxide (CO2), Argon (Ar), etc. Each element and compound exists separately and independently of all the other elements and compounds in the mixture. This is like a mixture og granules of salt and pepper.
Dalton's Law consists of three parts: (1) each gas in a mixture of gases exerts it's own (partial) pressure, (2) the (partial) pressure exerted by each gas in the mixture is independent of the presence of all the other gases in the mixture, (3) the total pressure exerted by the mixture of gases is equal to the sum of the (partial) pressures exerted by each gas in the mixture.
Pr (Air) = Pr (N2) + Pr (O2) + Pr. (H2) + Pr (CO2) + Pr (Ar) + -- + Pr (water vapor, or steam)
Pr (Air) = Pr (water vapor, or steam) + Pr (dry air, or all the other gases)
Properties of Dry Air andMoisture Saturated Air
Properties of Dry Air and Moisture Saturated Air
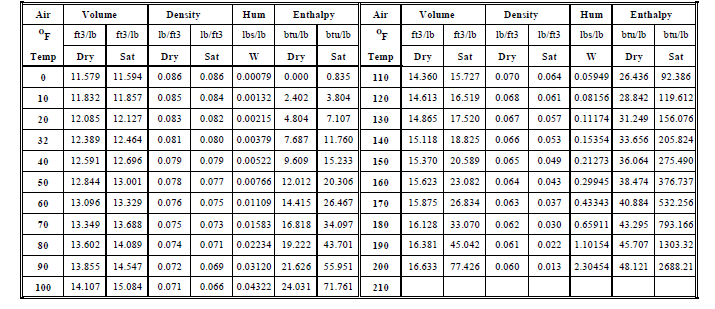
Table -
Specific Volume (V) is the volume occupied by 1 lb of air. Density (d) is the weight of 1 cubic foot of air. Dry Air has no moisture (water vapor) in it. Saturated Air has maximum possible moisture for the given temperature. The Relative Humidity (RH) is 0% when the air is completely dry and it is 100% when the air is saturated with moisture.
Humidity Ratio (W) shown is the amount (weight in lbs) of moisture in 1 lb of saturated air at the given temperature. Enthalpy (H) of Dry Air is the amount of heat (measured in btu from 0 oF) in 1 lb of air = M_da * spht * ( t -0 ) = H_da. Enthalpy of moist air is the enthalpy of dry air + latent heat to evaporate moisture in the air = H_da + M_w * LH.
In most calculations the density of air that is used is 0.075 lbs of air in 1 cubic foot volume of air. This corresponds to the density of dry air at 70oF and the density of saturated air at 60oF. Note that the density of dry air at 0oF is 0.086 which is 15% heavier than dry air at 70oF (0.086/0.075= 1.15). The density of dry air at 120 oF is 0.068 which is 10% lighter than dry air at 70 oF (0.068/0.075 = 0.90).
Heavier or denser air will try to move into a space with lighter air. So in winter the outdoor air at 0oF will try and infiltrate into the enclosed space maintained at a higher temperature of 70oF. Infiltration in winter can be a major heating load if cracks around windows and doors are large. In summer you can have exfiltration from mechanically cooled enclosed spaces but the amount of energy wasted is usually negligible.
Water Vapor in Air
The relative weight of water vapor in 1 lb of air is extremely small. This moisture together with the heat content of the moisture has a significant impact on human comfort conditions, At 75oF and 50% RH (relative humidity) the amount of water vapor in 1.0 lbs of air is 0.009 lbs. Since this unit is very small, another unit called grains of moisture is used where 1 lb water vapor = 7000 grains of water vapor.
Consider the heat content (H btu per lb of Dry Air) and moisture content (W grains of water vapor per lb of Dry Air) at different temperatures and relative humidities as shown in Table-x.
Moisture and Heat Content in 1 lb of Air at different Temperatures & Relative Humidities

Table -
A temperature of 75oF (24oC), 50% RH at standard pressure of 14.7 psia is considered comfortable. The heat content of this air is 28.3 btu/lb. At 105oF, 10% RH the heat content is about the same at 29.6 btu/lb and considerably less than 75oF, 100% RH. Very low moisture quantities in air (not necessarily low relative humidity of air) are also uncomfortable. Note that the moisture content of 105oF, 20% RH is 68 grains/lb and is in the same range as 65 grains/lb of 75oF, 50% RH. A relative humidity of 0 % at any temperature is uncomfortable for breathing.
The heat content of the air determines comfort conditions and comfort conditions can be achieved at higher temperatures by lowering the moisture content or relative humidity. The vapor pressure in the air depends on the amount of water vapor in the air. Water vapor (steam in the air) travels from areas of higher vapor pressure to areas of lower vapor pressure. This happens through air and also through other materials such as porous concrete and brick walls. In fact porous materials might prevent dry air from travelling through it, but it will allow moisture to travel through it if there is a difference in vapor pressure on each side.
The specific heat of "dry air" at constant pressure is approximately 0.24. However moist air includes water vapor with a specific heat of 0.45, and the specific heat of moist air is the weighted average of the specific heats of dry air and water vapor. The moisture content of 1 lb of moist air at 95 oF and 70% RH is 0.0254 lbs. The weighted average is 0.0254*0.45 + (1-0.0254)*0.24 = 0.2453. At 60oF and 10% RH the water vapor weight is 0.0012 lbs and the weighted average specific heat is 0.2402.
Steam
Besides air and water, the medium that is used extensively in building environmental control is steam. Substances like aluminum in their natural state, or at normal atmospheric pressures and temperatures, are solids. If you add heat to a solid and increase it's temperature then at some point it will start to melt into a liquid. Additional heat will make it boil or evaporate into a gas. The two transitional phases of solid to liquid and liquid to gas consists of adding heat without an increase in temperature. The reverse process of removing heat will make the substance go from gas to liquid and then liquid to solid. Water in it's natural state is a liquid. But water as a solid (ice) and gas (steam) also have major applications in HVAC systems.
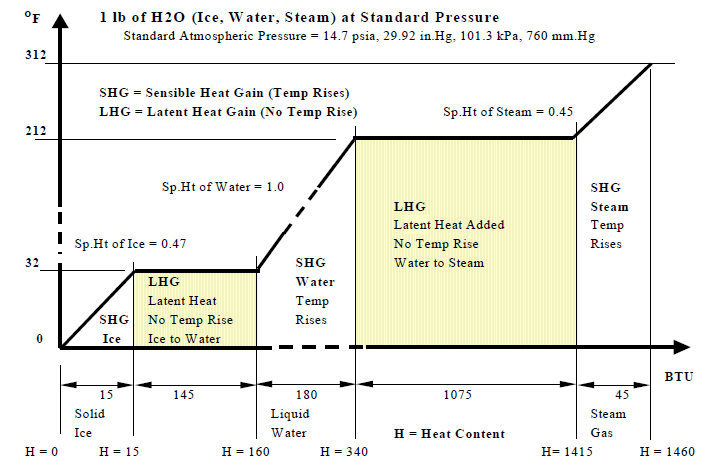
Figure -
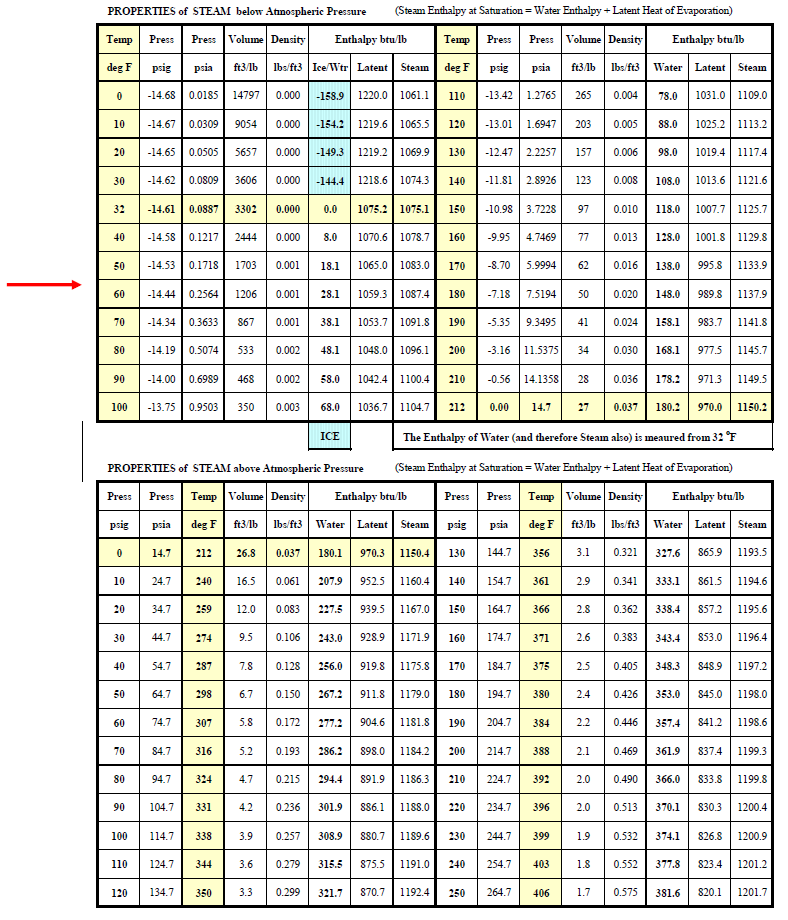
Table -
Figure ?? shows the process at standard atmospheric pressure of 14.7 pounds per square inch absolute (psia). Ice melts at 32oF and water boils at 212oF. The table below shows that these properties vary with pressure. At 250 psig (160.7 psia) water boils at 460oF. At 900 psig water boils at about 535oF. So it is possible to have water at temperatures above 212oF. Medium temperature (up to about 250oF) and high temperature (up to 400oF) hot water is used for district energy distribution.. conditions and hot water distribution temperatures within the building range from 100oF to 200oF.
Sensible Heat is the heat gained or lost by a substance without a change of state from solid to liquid or liquid to gas. There is always a change in temperature. Latent Heat is the heat that changes the state of a substance from solid to liquid or liquid to gas without a change in temperature.
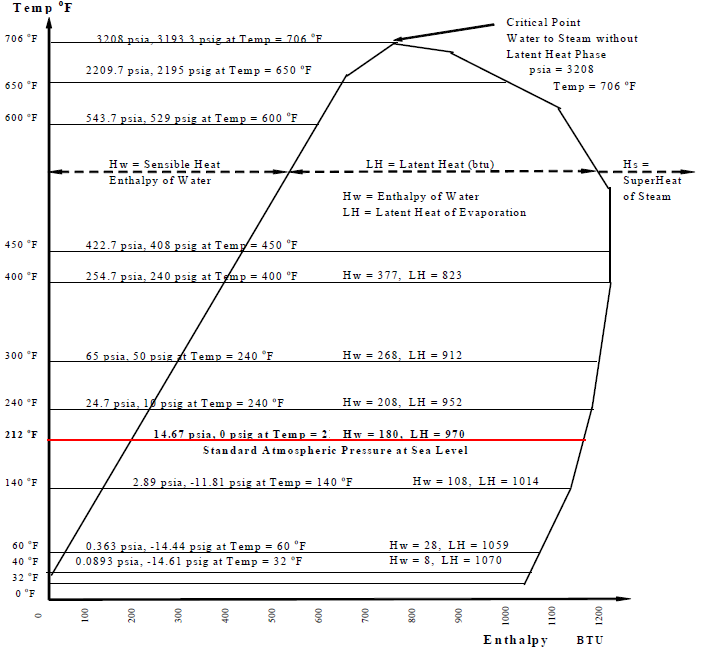
Figure -
Enthalpy (H)
The term heat was explained earlier. The measure of heat in I-P units was established as the amount of heat required to raise 1 lb of water through 1oF. So this also establishes the specific heat of water as 1.0. The amount of heat required to raise 1 lb of ice through 1oF is 0.47 btu, so the specific heat of ice is 0.47. Since the specific heat of ice is about 0.47, the heat content increase of 1 lb of ice from 20oF to 30oF is 4.7 btu. Similarly the specific heat of steam is 0.45 and the heat content increase of 1 lb of steam from 210oF to 220oF is 4.5 btu.
Enthalpy is a measure of the heat content of a substance. Like all measuring systems, an arbitrary zero level and scale were established for measuring enthalpy. The enthalpy of water and steam are measured from 32oF. So the enthalpy of water below 32oF (which is ice at standard pressure) have negative values. Water at 32oF has 0 enthalpy. It requires about 144 btu to convert 1 lb of water to ice, so ice at 32oF has -144 btu (negative 144 btu). There is actually no such thing as negative heat content and it only exists theoretically because of the arbitrarily selected zero point. If heat content was measured from absolute zero temperature (0oR or -460oF) then all enthalpy values, or heat contents of substances, would be positive.
The enthalpy of air is measured from 0oF. So the enthalpy of humid air has two components. The water/steam part is measured from 32oF and the dry air part is measured from 0oF. At 0oF, most of the water vapor part of the air has condensed out and solidified into ice and the air can be considered to be dry. However, as explained earlier, the air at 0oF can still contain water vapor because the partial pressure of the water vapor in the air might be say 0.02 psia and total pressure of the water vapor mixture adds up to 14.7 psia. In the S-I or metric system the enthalpy of both air and water vapor is measured from 0oC (the freezing point of water).
Atmospheric pressure decreases with altitude. The pressure in Boulder Colorado at 6,000 feet elevation is 11.8 psia and at this pressure water boils at about 210oF. La Paz, the capital City of Bolivia in South America is at an elevation of 12,000 feet and atmospheric pressure is 9 psia and water boils at 190oF. The top of Mt. Everest is 30,000 feet and the pressure is 4 psia and water boils at 155oF. The density of air and the quantity of oxygen in the air decreases with increasing altitude. The properties of air and water vary with altitude and affect the performance of air handling equipment, cooling equipment and combustion equipment (includes automobile engines) and all engineering equipment have to be configured, adjusted and selected for altitude.
The symbol and abbreviation for enthalpy is H. Figure ? is a Temperature – Enthalpy chart. It gives us a picture of the behavior of water and steam under varying pressures.
Example-1: What is the enthalpy of 1 lb of steam at 312oF, measured from 0oF, at standard pressure of 14.7 psia? See Figure ?.
- The heat required to melt 1 lb of Ice at 32oF to 1 lb of Water at 32oF is 145 btu.
- The heat required to boil water at 212oF to Steam at 212oF is 970 btu.
- The specific heat of Ice is 0.47, of Water is 1.0, and Steam is 0.45.
- H1 = Ice from 0oF to 32oF = 1 lb x 0.47 SpHt x (32oF - 0oF) = 1 x 0.47 x 32 = 15 btu.
- H2 = Latent Heat to melt Ice at 32oF into Water at 32oF = 1 lb x 145 = 145 btu.
- H3 = Water from 32oF to 212oF = 1lb x 1.0 x (212oF - 32oF) = 1 x 1.0 x 180 = 180 btu.
- H4 = Latent Heat to boil Water at 212oF into Steam at 212oF = 1 lb x 970 = 970 btu.
- H5 = Steam from 212oF to 312oF = 1 lb x 0.45 SpHt x (312oF - 212oF) = 45 btu.
Enthalpy (H) = H1 + H2 + H3 + H4 + H5 = 1355 btu for 1 lb
Example-2: What is the enthalpy of 1 lb of steam at 312oF, measured from 32oF, at 14.7 psia?
Enthalpy (H) = H3 + H4 + H5 = 1195 btu. This is the official H of steam at 312oF.
Example-3A: What is the enthalpy of Air at 105oF and Relative Humidity of 100% at standard pressure of 14.7 psia? See Table ?
The enthalpy of dry air measured from 0oF has to be added to the enthalpy of the moisture (water vapor or steam) content of the air measured from 32oF. The specific heat of air is 0.24, of water is 1.0 and the latent of evaporation of water to steam is 1035 btu.
1 lb of moist air at 105oF and RH of 100% consists of 0.051 lbs of water vapor or steam (W-w) and 0.949 lbs of dry air (W-a). W= 0.051 lbs + 0.949 lbs = 1.0 lb.
- H-a = 0.949 lbs x 0.24 spht x (105oF - 0oF) = 23.915 btu
- H-w = 0.051 lbs x 1.0 spht x (105oF - 32oF) = 3.723 btu
- H-lh = 0.051 lbs x 1035 LH = 54.825 btu
- H = H-a + H-w + H-lh = 81.7 btu ( see Table ?. The difference is due to the accuracy level).
Example-3B: What is the enthalpy of Air at 105oF and RH = 50%? See Table ?
- W-w = 0.025 lbs and W-a = 1 - 0.025 = 0.975 lbs
- H-a = 0.975 x 0.24 x (105 - 0) = 24.57 btu.
- H-w = 0.025 x 1.0 x (105 - 32) = 1.825 btu.
- H-lh = 0.025 x 1075 = 26.875 btu
- H = 24.570 + 1.825 + 26.875 = 53,27 btu
Example-3C: What is the enthalpy of Air at 105oF and RH = 0%? See Table ?
- W-w = 0, H-w = 0 and H-lh = 0
- H-a = 0.975 x 0.24 x (105 - 0) = 24.57 btu.
- H = H-a = 24.57 btu.
Example-4: What is the enthalpy, measured from 0oF, of 1 lb of Aluminum at 200oF? The specific heat of aluminum is 0.22.
The example states that the enthalpy of aluminum is to be considered as zero at 0oF. The heat content or enthalpy of aluminum at 200oF is = M x spht x DT = 1 lb x 0.22 x (200 - 0) = 44 btu.
Work
Work measurement was established arbitrarily and by tradition in the same way as length, weight, time, heat, temperature and pressure. In I-P units a unit of work is done when 1 lb mass is lifted vertically against gravity through a distance of 1 foot. The unit is called foot-pound (ft-lb). Similarly, when 1 kilogram mass is lifted vertically against gravity through a distance of 1 meter then the work done is 1 kilogram-meter.
1 lb weight resting on a surface area of 1 square foot exerts a pressure or force of 1 lb per square foot. Supposing the surface area of the soles of a person’s two feet is 1 square foot (each foot being 12 inches by 6 inches) and the person weighs 100 lbs then the person exerts a force of 100 lbs/ft2 on the ground. If you lift this person off the ground by 1 foot then you have done 100 ft-lbs of work. Work done is Force x Distance.
Force is pressure per unit area. In Figure ?? force, behind a piston, is applied to a gas in a cylinder, moving the cylinder a certain distance and compressing the gas. So work has been done. Figure ?? shows that the work done is the area under the curve from pressure-volume condition 1 to pressure-volume condition 2. The process is not usually a straight line.

Figure -

Figure
Energy
Energy is defined as the capacity to do work. So energy can exist in many forms such as nuclear, chemical, electrical, fossil fuel, solar, wind and geothermal. The basic forms of energy are potential, kinetic, mechanical, heat, internal, and electrical.
Potential energy is due to position or state. For example a mass raised to some height above a reference level such as the earth's surface can be made to do work by letting it fall. A compressed spring possesses potential energy because it can do work when it is released.
Mechanical energy is possessed by any mass which is in motion. It is also referred to as kinetic energy. Kinetic energy is the energy possessed by a body in motion. Electrical energy is obtained from electrical generators and batteries and consists of the flow of electrons in an electrical circuit.
Heat energy is the result of the kinetic energy possessed by the atoms and molecules which make up the mass. Internal energy of a gas is a function of temperature only and is independent of changes in pressure and volume and the symbol used is U. This is known as Joule's Law. In general for solids, liquids and gases, a change in internal energy (dU) is related to the change in heat content (dQ) and the change in work done (dW).
dQ = dU + dW
When one mass loses X btu of heat then the heat content of other masses must increase by the same X btu. There is no such thing as heat or any form of energy just disappearing into nothing. Energy is transferred from one object to another or just stays where it is.
Energy can be converted from one form to another. The heat energy from fossil fuel can be converted into mechanical energy to drive turbines. The mechanical energy of turbines can be converted to electrical energy using generators. Electrical energy can be converted to mechanical energy using motors or converted to heat energy as in the case of resistance heaters.
This is the basis of the First Law of Thermodynamics which states that heat and work are interconvertible. If any system goes through a process during which work energy or heat energy is added or removed from the system, then none of the energy added is destroyed within the system and none of the energy removed is created within the system. In other words energy is indestructible and the total energy (in the universe) remains the same.
Another observed phenomenon is that heat will not of it's own accord flow up the gradient of temperature. For example if one object is at 100oF and another at 10oF then heat will flow from the object at 100oF to the object at 10oF and not the other way around. It can be made to do so by applying some form of external energy such as mechanical energy as in the case of refrigeration equipment. However, external energy is not required for the transfer of heat from an object at a higher temperature to an object at a lower temperature as in the case of heating equipment. This is basis of the Second Law of Thermodynamics which states that heat will not flow up the gradient of temperature.
Neither of the Laws of Thermodynamics can be proved, but since no exceptions to the two laws have ever been observed, they are accepted as true.
The main use of Energy is used to do work. For example the heat energy from petrol is converted to mechanical energy to move an automobile. The process is reversible. Mechanical energy can be converted to heat energy. For example the mechanical energy applied to a rotor churning a liquid in a barrel is converted to heat energy and this will raise the temperature of the liquid.
A scientist named James Joule proved that the ratio of work done to heat generated is constant.
- W / Q = J
- W = mechanical work done in work units (ft-lbs)
- Q = heat generated in heat units (btu)
- J = constant. J is called Joule's Mechanical equivalent of Heat
- It has been proved that 778 ft-lbs of work will generate 1 btu of heat.
- So J = W/Q = 778 / 1 = 778 ft-lbs
Example of the Use of Charts: The Refrigeration Cycle
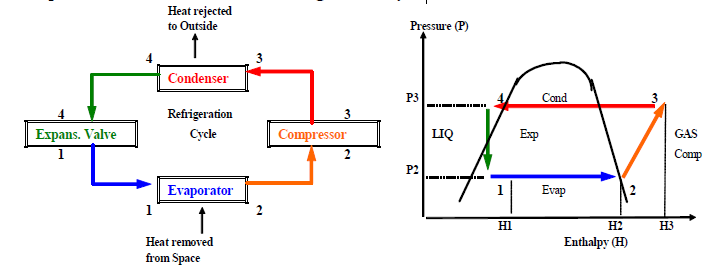

Figure
Adiabatic and Isothermal Processes
The adiabatic process is essentially one where no heat is added or removed. In the P-H chart of Figure ??, the process through the expansion valve from 4 to 1 is adiabatic. The process is instantaneous and there is no means for heat gain from the surrounding space or loss to the surrounding space. The compression process from 2 to 3 can also be considered adiabatic. The piston compresses the gas in the engine cylinder in a fraction of a second. However, the gasrefrigerant does increase in enthalpy as shown in the P-H chart. The gain in enthalpy is the heat equivalent of the mechanical energy applied to the piston to compress the gas.
The isothermal process occurs at constant temperature. In the evaporator, the liquid refrigerant absorbs heat from the space being cooled and is converted to gas. The process consists of latent heat gain that vaporizes the liquid which occurs at constant temperature. In the condenser, the refrigerant gas is cooled and condenses into liquid. Latent heat is removed at constant temperature to convert gas to liquid. Note that the Pressure-Volume compression and expansion processes are not straight lines.
Power
The same amount of work can be done over different time periods. Moving 1000 lbs through a distance of 1000 feet in 100 hours is not the same as moving the same 1000 lbs through the same distance of 1000 feet in 1 hour. Power is defined as work done per unit time or the rate at which work is done.
A unit of work commonly used in I-P units is the Horse-Power (HP) which is defined as 33,000 foot-pounds per minute or 550 foot-pounds per second. This is used in mechanical engineering calculations. The electric HP is equivalent to 33,013.282 ft-bs and boiler HP is 33,472.12 ft-lbs. The metric HP is 32,548.5623 ft-lbs per minute or 542.476 ft-lbs per second.
However the unit of power commonly used in S-I units is the watt which is derived from electrical units. Electrical force (E volts) = Current (I amperes) x Resistance (R ohms) and Power (P watts) = E x I. The watt is a small unit so the term corresponding to HP is Kilowatts (KW) where 1 KW = 1000 watts. 1 KW= 44,253.7 ft-lbs = 1.340483 HP and 1 HP = 0.746 KW.
